Rizmoic®
COMPOSE-1 og 2: Studie av naldemedins effekt ved opioidindusert obstipasjon
Opioid-induced constipation (OIC) remains a significant challenge in the management of chronic non-cancer pain, affecting patient compliance and quality of life. Naldemedine, a peripherally acting μ-opioid receptor antagonist, offers a promising solution by selectively blocking opioid effects in the gastrointestinal tract without impacting central analgesia. This article delves into the results of two pivotal phase 3 trials, COMPOSE-1 and COMPOSE-2, evaluating the efficacy and safety of naldemedine in managing OIC.
Study design
Both COMPOSE-1 and COMPOSE-2 were multicenter, phase 3, randomized, double-blind, placebo-controlled trials conducted across several countries including the USA and parts of Europe. The primary objective was to evaluate the efficacy of naldemedine compared to placebo in alleviating constipation symptoms in adults receiving stable opioid dosages for chronic non-cancer pain, who had discontinued all other laxative treatments. The primary endpoint was the proportion of responders, defined by an increase in weekly spontaneous bowel movements (SBMs).
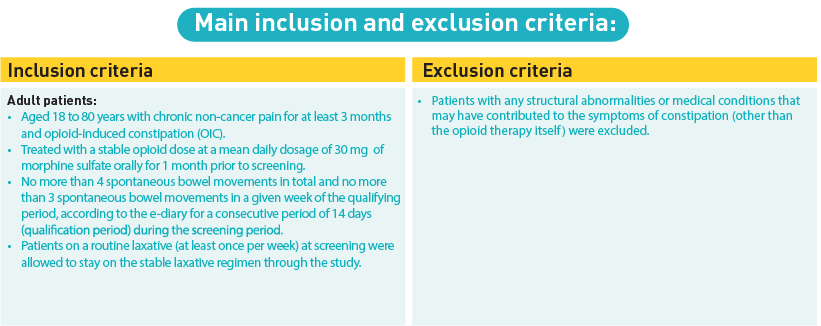
Participants were adults aged 18 to 80 years who had been on a stable opioid regimen and exhibited fewer than four spontaneous bowel movements per week, among other criteria. They were randomized in a 1:1 ratio to receive either 0.2 mg of naldemedine or a matching placebo once daily for 12 weeks, following a 2–4 week screening phase. Randomization was stratified based on the average total daily opioid dose into two categories: 30–100 mg and over 100 mg of oral morphine sulphate equivalents.
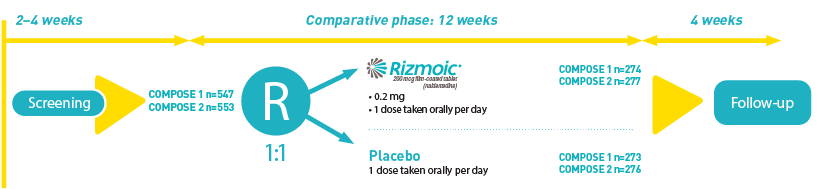
The trials collectively enrolled over 1,100 participants, with COMPOSE-1 and COMPOSE-2 including 547 and 553 patients respectively. These participants had demonstrated clinical signs of OIC and had not used any laxatives or were willing to discontinue such treatments during the study period.
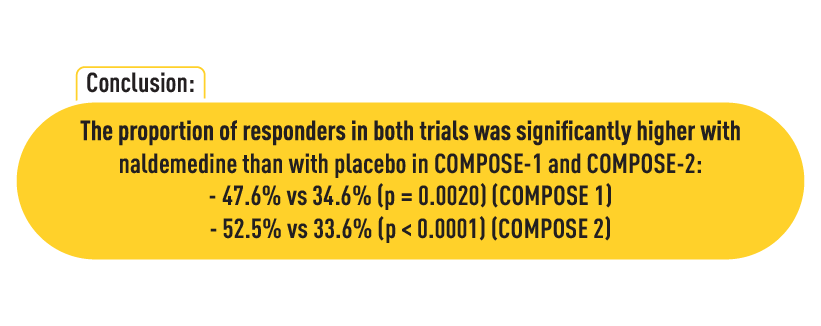
Efficacy outcomes
The primary efficacy endpoint was the percentage of patients achieving at least three spontaneous bowel movements per week, with an increase of at least one bowel movement per week over baseline for at least 9 of the 12 weeks of the trial, including 3 of the last 4 weeks.
- COMPOSE-1: The naldemedine group showed a significantly higher percentage of responders (47.6%) compared to the placebo group (34.6%), with a statistical significance of p=0.0020.
- COMPOSE-2: Similarly, a higher responder rate was observed in the naldemedine group (52.5%) compared to placebo (33.6%), with p < 0.0001.
These results highlighted the effectiveness of naldemedine in improving bowel function among patients suffering from OIC due to opioid therapy.
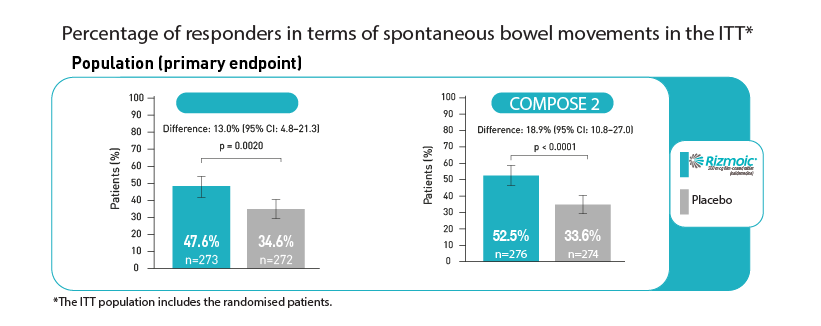
Adapted by Viatris from Hale et al (2017)
Safety and tolerability
The safety profile of naldemedine was closely monitored throughout the trials, with particular attention given to treatment-emergent adverse events (TEAEs). The most common TEAEs included gastrointestinal symptoms such as abdominal pain and diarrhea, which were generally mild to moderate in severity.
- In COMPOSE-1, 49% of the naldemedine group experienced any TEAEs, compared to 45% in the placebo group.
- In COMPOSE-2, these figures were 50% for naldemedine and 48% for placebo.
The incidence of serious TEAEs was low and comparable between the naldemedine and placebo groups across both trials. Importantly, there was no significant impact on opioid-mediated analgesia, nor was there a notable increase in opioid withdrawal symptoms, underscoring the peripheral specificity of naldemedine.
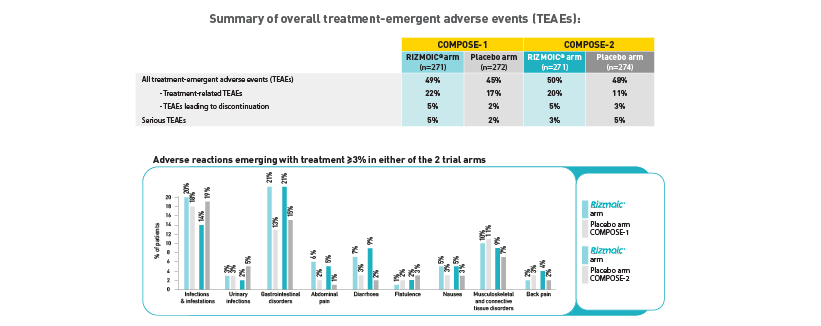
Created by Viatris based on Hale et al (2017)
Clinical implications
The results from COMPOSE-1 and COMPOSE-2 suggest that naldemedine is an effective and safe option for the management of OIC in patients with chronic non-cancer pain. Its ability to improve bowel function without affecting central opioid effects represents a significant advantage over traditional laxatives, which do not target the underlying pathophysiology of OIC.
For healthcare professionals managing patients with chronic pain, naldemedine offers a clinically validated option to alleviate the burdensome symptoms of OIC. Its once-daily oral dosing can be easily integrated into most patient routines, potentially improving compliance and overall quality of life. Yet, it is important to consider individual patient profiles and potential interactions with other medications due to naldemedine's mechanism of action involving P-glycoprotein substrates.
Conclusion
Naldemedine has been shown to relieve constipation in patients following opioid treatment for chronic non-cancer pain. The COMPOSE-1 and COMPOSE-2 trials underscore its efficacy and safety profile, supporting its use as a targeted treatment option that can improve patient outcomes and quality of life as discussed in Camilleri's paper. Healthcare providers should consider naldemedine as a key component of the therapeutic arsenal for managing OIC in patients undergoing long-term opioid therapy for non-cancer pain.
NO-RIZM-2024-00001 | 11-2024
Reference
- Hale M, Wild J, Reddy J, et al. Naldemedine versus placebo for opioid-induced constipation (COMPOSE-1 and COMPOSE-2): two multicentre, phase 3, double-blind, randomised, parallel-group trials. Lancet Gastroenterol Hepatol. August 2017; 2(8):555–564
- Camilleri M, Hale M, Morlion B, et al. Naldemedine Improves Patient-Reported Outcomes of Opioid-Induced Constipation in Patients with Chronic Non-Cancer Pain in the COMPOSE Phase 3 Studies. J Pain Res. 2021 ; 14:2179-2189
Mer informasjon om
Bivirkninger av opioider

Omfattende beskrivelse av opioiders bivirkninger og smertestillende bivirkninger.
Viktige forskjeller mellom OIC og funksjonell forstoppelse

Lær mer om forskjellene mellom opioidindusert og funksjonell forstoppelse, hvordan de virker, symptomer og behandling for å forbedre pasientbehandling.